
Universal indicator changes its color depending on the pH of the solution. Here universal indicator has been added to several vials containing solutions with varying pH values, left to right: from pH=2 to pH=12 with a step of pH=1 (green pH=7 is in the center).

A mixture of aluminum (Al) metal powder and iron oxide (Fe2O3) powder is placed on top of a beaker filled with sand. The mixture is then lighted. The thermite reaction is highly exothermic, it produces a lot of heat and sends out sparkles of molten iron: Fe2O3 + Al -> Fe + Al2O3.

Calcium metal (Ca) reacts vigorously with water (H2O), producing hydrogen gas (H2) bubbles and slightly soluble calcium hydroxide (Ca(OH)2): Ca + H2O -> Ca(OH)2 + H2. In this sequence a chunk of calcium is dropped into a beaker with water. Calcium first sinks but then bubbles up due to H2. Calcium hydroxide is seen as white precipitate.

Phenol red indicator changes its color depending on the pH of the solution. Here phenol red has been added to three beakers containing solutions with different pH values, front to back: acidic, neutral, and alkaline.

A couple of drops of glycerin (C3H8O3) are placed onto a pile of potassium permanganate (KMnO4). In this exothermic reaction glycerin bursts into flames and white smoke appears. The redox reaction is: KMnO4 + C3H8O3 -> K2CO3 + Mn2O3 + CO2 + H2O.

The Erlenmeyer flask contains sulfuric acid (H2SO4) with a couple of drops of phenolphthalein added. A solution of sodium hydroxide (NaOH) is run from the Burette until the solution in the flask becomes pink.

Sulfur powder in a deflagration spool is burning in air. It produces a bright blue flame. Sulfur dioxide gas (SO2) is the main product of the combustion reaction: S + O2 -> SO2. This is an example of a synthesis reaction.

Steel wool (25-micron thin bundled steel filaments) is burning in an Erlenmeyer flask filled with chlorine (Cl2) gas. Steel is mostly iron (Fe). Yellow-brown iron(III) chloride (FeCl2) is produced: Fe + Cl2 -> FeCl3.

Hot molten sulfur is poured from a test tube into a beaker with cold water. When rapidly cooled, molten sulfur turns into an elastic solid, known as "plastic" or amorphous sulfur.

A test tube with powdered sulfur, S, is placed over a Bunsen burner flame. When heated, it first melts to a straw-yellow liquid and then darkens to a red-brown color.

Brass fitting is placed in a beaker with concentrated nitric acid (HNO3). Brass is an alloy of copper (Cu) and zinc (Zn). Copper in the brass is oxidized to form copper nitrate (Cu(NO3)2) and brown nitrogen dioxide gas (NO2): Cu + HNO3 -> Cu(NO3)2 + NO2 + H2O.

Several pieces of white dry ice are placed in a watch glass in a ring stand. Dry ice is solid carbon dioxide (CO2). Under normal conditions, it sublimes, i.e. turns to gas directly from solid state. White smoke is a fog or mist of water droplets condensed from the air.

Copper metal wire coil was placed into a test tube containing a 0.25M silver nitrate solution. Since copper is more reactive than silver, a single-displacement reaction occurred: Cu + AgNO3 -> Ag + Cu(NO3)2.

A small piece of sodium metal (Na) burns in an Erlenmeyer flask filled with chlorine gas (Cl2). The reaction produces bright yellow light and white fumes of sodium chloride (NaCl): Na + Cl2 -> NaCl.

Steel wool is made of very thin bundled steel filaments (25 micron thickness here). Steel wool burns in air (20% oxygen by volume) only gently. In this oxidation reaction a mixture of different iron oxides is produced (FeO, Fe2O3, Fe3O4), depending on how much oxygen gets to a particular bit of iron.

A piece of limestone (mineral of calcium carbonate, CaCO3) is heated in the flame from a propane Bunsen burner. When heated to 900-1200C, calcium carbonate decomposes into quicklime (calcium oxide, CaO) and carbon dioxide gas (CO2): CaCO3 -> CaO + CO2. Calcium gives the flame a bright orange-yellow color.

Silver chromate (Ag2CrO4) precipitate (bright red) formed by adding 0.25 M solution of silver nitrate (AgNO3) to 0.25 M solution of potassium chromate (K2CrO4). The reaction is K2CrO4 + AgNO3 -> Ag2CrO4 + KNO3.

Dry ice is solid carbon dioxide (CO2). When dropped into water, it sublimes, i.e. turns to gas directly from solid state. Some carbon dioxide forms weak carbonic acid with water, H2CO3. Here water has universal indicator added. It changes the color from green (neutral) to orange-red, signifying acid formation. White smoke is a fog or mist of water droplets.

Powdered aluminum metal (Al) and iodine (I2) are mixed together and placed in a watch glass. A drop of water is used to initiate the synthesis reaction: Al + I2 -> Al2I6. It is an exothermic redox reaction that produces a lot of heat so that iodine sublimates and forms a violet vapor.

Flame tests for different elements, left to right: strontium, copper, potassium, calcium. Nichrome wire loop is dipped into a solution of the respective compound and brought into the flame from propane Bunsen burner. The color of the flame is determined by the element's emission spectrum which is characteristic to that element. Therefore, flame test can be used as an analytic procedure for identifying elements.

When 0.25 M solution of silver nitrate (AgNO3) is added 0.25M solution of potassium bromide (KBr), a pale cream silver bromide (AgBr) precipitate is formed. The reaction is: KBr + AgNO3 -> AgBr + KNO3. This double displacement reaction can be used as a test for presence of bromides in a solution.

Gas diffusion is demonstrated using nitrogen dioxide (NO2) gas. Nitrogen dioxide is heavier (more dense) than air and has a characteristic brown color. A jar filled with nitrogen dioxide is placed under an inverted glass (left frame). After several minutes the gas uniformly fills the whole volume under the glass (right frame).

A droplet of water (H2O) is dropped onto a small piece of sodium metal (Na) in a deflagrating spoon. Sodium reacts exothermically with water: Na + H2O -> NaOH + H2. Due to released heat, sodium melts, catches fire and starts to burn with characteristic yellow-orange flame. A mixture of sodium oxide (Na2O) and sodium peroxide (Na2O2) is produced: Na + O2 -> Na2O and Na + O2 -> Na2O2.

Corn oil is being poured into a cup with water. Oil and water are mutually insoluble (immiscible) and oil is less dense than water.

Lead (II) nitrate (Pb(NO3)2) is poured into a beaker containing potassium iodide (KI). Both solutions are clear and have 0.2 M concentrations. Yellow lead (II) iodide precipitate (PbI2) is formed: KI + Pb(NO3)2 -> PbI2 + KNO3. This is an example of a double displacement reaction.

A small piece of sodium metal (Na) is dropped into a Petri dish with water (H2O). Sodium melts, moves around and fizzes vigorously, producing bubbles of hydrogen gas (H2) and sodium hydroxide (NaOH): Na + H2O -> NaOH + H2. The presence of the hydroxide is accentuated by the addition of few drops of phenolphthalein indicator, which turns the solution pink, indicating alkaline solution.
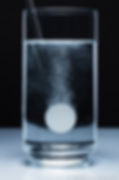
Alka-Seltzer is an effervescent antacid and pain reliever. It contains three active ingredients: aspirin, sodium bicarbonate, and citric acid. When dissolved in water, sodium bicarbonate and citric acid react to produce bubbles of carbon dioxide gas: H3C6H5O7 + NaHCO3 -> Na3C6H5O7 + CO2 + H2O.

Left: red copper (I) oxide, Cu2O, right: black copper (II) oxide, CuO, in beakers.

A small mound (3.6 g) of ammonium dichromate ((NH4)2Cr2O7) is placed on a ceramic tile. When ignited, ammonium dichromate decomposes, gives off orange sparks and throws up green chromium(III) oxide, producing an effect resembling a volcano. The reaction is (NH4)2Cr2O7 -> Cr2O3 + N2 + H2O.

A bar magnet is used to separate iron metal powder (Fe) from its mixture with sulfur powder (S). Constituents in a mixture are not chemically bonded to each and retain their individual properties. Here iron is ferromagnetic and is attracted to a magnet while sulfur is not. Therefore they can be physically separated with a magnet.

Zinc metal (Zn) reacts with 3M hydrochloric acid (HCl) in a test tube. The reaction produces hydrogen gas (H2) bubbles: Zn + HCl -> ZnCl2 + H2. This is an example of a single displacement reaction.

Magnetite is a mineral of iron oxide (Fe3O4, iron(II,III) oxide). It is a naturally-occurring ferromagnetic and can be magnetized to become a permanent magnet. Naturally-magnetized magnetite is called lodestone. Here a piece magnetite is shown to attract steel paper clips and safety pins.

Reaction rate increases with concentration of reactants. This effect is demonstrated here using the reaction of chalk (calcium carbonate, CaCO3) with hydrochloric acid (HCl). Carbon dioxide (CO2) bubbles are produced: CaCO3 + HCl -> CaCl2 + H2O + CO2. In the left beaker the concentration of hydrochloric acid is 0.5M, while in the right beaker the concentration is 0.2M. The reaction is visibly more vigorous (higher reaction rate) in the left beaker.

Magnesium coils are placed in test tubes with hydrochloric acid of different concentrations at room temperature: 1 M (left), 0.1 M (center), and 0.01 M (right). Magnesium reacts with the acid producing hydrogen bubbles: Mg + HCl -> MgCl2 + H2. The reaction proceeds more vigorously with increasing concentration of the acid. This is an example of a single displacement reaction.

Elemental gallium (Ga) has a melting point of 29.76 C. Here solid gallium ingot is placed in the palm of a gloved hand. Body heat leads to melting of the ingot.

Two solutions are added to a beaker to form separate layers, 1,6-hexanediamine (bottom) and sebacyl chloride/hexane (top). A film of synthetic polymer Nylon 6-10 is formed at the interface of the two solutions. The film is snagged and slowly pulled out from the beaker to form a long strand. Continuous strand of Nylon is then wrapped around the glass rod.
